We are in a golden age of outdoor and camp cooking, with each year seeing new companies starting to produce quality cookware at great prices. The problem is, with all of these new companies pining for us to buy their products, buying a new set of camp cookware can be confusing. Let’s cut through the hype and dig deep into the material science behind many of these cookware designs. Doing so will allow us to see the pros and cons of each material and help ensure you choose the cookset with the right material for your cooking needs. I am going to apologize now for, as you will soon read, my geeking out about this topic. I will freely admit that I am a nerd and, if you have attended one of our cooking classes, tend to get overly excited about the science that is going on behind the scene.

Resistance to heat transfer also means they are prone to hot spots. This is due to the material’s inability to conduct heat through itself and away from the heat-source. Cookware manufactures can combat hot spots by increasing the material thickness. The increased thicknesses essentially dissipating the heat over a larger area. This is a double-edged sword, however, as the greater the thickens the more energy is needed to heat the cooking surface.
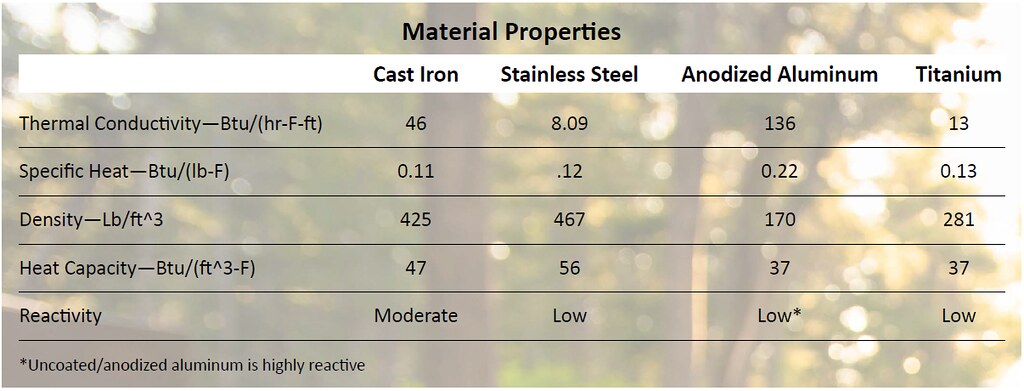
Now that we understand how heat transfer from the flame-side to the cooking side of a pot or pan we must think about how that heat is retained in the material. A pot or pan’s heat capacity is a function of the its material density and the specific heat. The material’s specific heat can be thought of as the amount of energy needed to increase the temperature of the material. When multiplied by the density we get a variable that helps us differentiate between the different materials.

Now that we understand how a material’s thermal properties can affect cooking performance we must turn our attention to an often overlook aspect, a material’s reactivity. Highly acidic ingredients or alkaline ingredients, tomatoes and onions (respectively), can create a chemical reaction with cookware material. This can cause the material to degrade and impart metallic flavors into the meal. Although the amount of metal that reacts with food and can be ingested is very small, there are some health concerns from prolonged exposure.
One way camping cookware manufacturers work around reactivity concerns is through the use of coatings like TeflonTM or enamel. These coatings generally serve two purposes when applied to the cooking surfaces. Firstly, they create a barrier between the reactive material and the food, preventing a chemical reaction from taking place. Secondly, they create a non-stick surface which allows food to brown without sticking.
Although widely used, coatings do have their limitations and are only as good as their adhesion to the base material. Excessive localized or prolonged exposure to heat can degrade this adhesion.

Additionally, coatings can be degraded by repeated abrasion. Once a coating is compromised, foods can begin to react with the base material, essentially eating underneath the coating. Eventually enough of the coating is detached from the base metal that if flakes off into the food. As with death and taxes, coatings are guaranteed to fail.
Conversely, nonferrous metals like aluminum and titanium can undergo a process called anodizing. Unlike coatings, which require adhesion to stick to the base material, the anodizing process converts the base material’s surface into an anodic oxide layer. Because the anodizing process chemically alters the base metal’s surface, it is less susceptible to mechanical and thermal degradation. This molecular alteration of the base material reduces the metal’s reactivity and improves the nonstick properties. Although the anodic oxide layer can eventually be compromised, anodizing is a great alternative to simply coating reactive materials with enamel or TeflonTM.
Now that we understand the science behind each of the material options let’s take a look at how the four most popular camp cookware materials measure up against each other.
Related Articles
The vast majority of our camp cooking is done using GSI’s Pinnacle line of cookware. It’s anodized aluminum body distributes heat uniformly across the cooking surface, even when cooking over the small burners found in modern cook stoves. The Pinnacle line also utilizes new Teflon coating technology on the cooking surface to produce a great nonstick surface. The aluminum’s exceptional thermal conductivity more than makes up for its relatively low heat capacity.

Although the majority of our camp cooking is done with the anodized aluminum cookware, there is still a special place in my heart for cast iron cookware. It is the style of cookware I grew up camping with and the material of choice for generations before me. Although it takes longer to heat up, and is prone to hot spots if heated too quickly, cast iron’s heft and heat capacity still makes it my go-to cookware material when I want to perfectly brown meat or saute vegetables.

So I guess, in a sense, there really isn’t one material that is the be all and end all for camp cookware. It would be easy to say, based on material properties, you should go and buy cookware made from a certain material but it isn’t that easy. Each one is really suited to different cooking needs. By selecting a material that best suits your cooking needs you can guarantee years of enjoyable camp cooking.
I would like to send a special thank you to Lodge Manufacturing Company and GSI Outdoors for answering all of my questions related to this article. We own, and continue to enjoy cooking with, equipment from both of these companies.
More Photos!
[flickr set=72157672871950164]
To get your copy of theSummer 2016 Issue: |









